Student Author: Patrick Sahrmann
Co-Authors: Kirklin McWhorter, Jessica R. Krewall, Dr. Douglas C. Goodwin
Catalase-peroxidase (KatG), an enzyme produced by bacteria and fungi, is especially prominent among some of the world’s most prolific pathogens (e.g., Mycobacterium tuberculosis). These organisms use KatG to defend against H2O2 produced by host immune responses. Contrary to all other members of its enzyme superfamily, KatG is bifunctional, capable of catalase and peroxidase activities. Further, against long-standing predictions that its two activities would be mutually antagonistic, we have shown that peroxidatic electron donors (PxEDs) stimulate KatG’s catalase activity.
A narrow active site access channel prevents PxEDs from directly reducing KatG’s heme cofactor, suggesting that the protein itself must facilitate free radical transfer from within the active site and channel this absence of an electron to the periphery of the enzyme via oxidizable amino acids (i.e., electron-hole hopping) to prevent irreversible inactivation). This links the active site to the solvent-accessible surface of KatG. In this manner, peroxidase activity (i.e., the use of PxEDs) serves to uphold KatG’s robust catalase activity. We have shown that one prominent electron hole-hopping pathway begins with oxidation of an active site tryptophan (W321). A methionine (M377), an amino acid which contains an oxidizable sulfur, 3.9 Å away from W321 is a prime candidate for the next step in this mechanism.
To investigate this possibility, we used site-directed mutagenesis to generate M377I KatG, replacing methionine with the non-oxidizable amino acid isoleucine. M377I KatG variant displays similar catalase activity to that of wild type, indicating M377 is not directly involved in catalase turnover. Subsequent additions of substrate after an initial reaction with H2O2 in the presence of ABTS, a peroxidatic electron donor, show a decreased initial rate, suggesting M377 is pertinent in the mechanisms of catalase recovery. M377I is incapable of aiding in through-protein radical transfer from W321, leading to irreversible inactivation of enzyme due to advanced oxidation of the protein even in the presence of ABTS. As with both wild-type and W321F KatG, a variant of KatG with the previously mentioned tryptophan replaced with a non-oxidizable phenylalanine, inclusion of a PxED sustains the catalase activity of the M377I variant to the complete consumption of H2O2. We propose that possible pathways for through-protein radical transfer expand as the distance from the active-site heme cofactor increases, thereby producing a corresponding decrease in contribution from any particular oxidizable amino acid in catalase activity preservation. Future research will focus on generation of more variants of KatG to identify amino acids involved in the hole-hopping mechanism, as well as a definitive look at the iron oxidation states of the heme cofactor using Mössbauer spectroscopy.
Statement of Research Advisor: Patrick Sahrmann’s research has helped to elucidate how redox enzymes protect themselves from inactivation by the highly reactive intermediates they must generate as part of the chemical reactions they catalyze. Redox enzymes are essential in every realm of biology, and the particular enzyme Patrick worked on is central to how pathogens like Mycobacterium tuberculosis defend themselves from host defenses. —Douglas Goodwin, Biochemistry
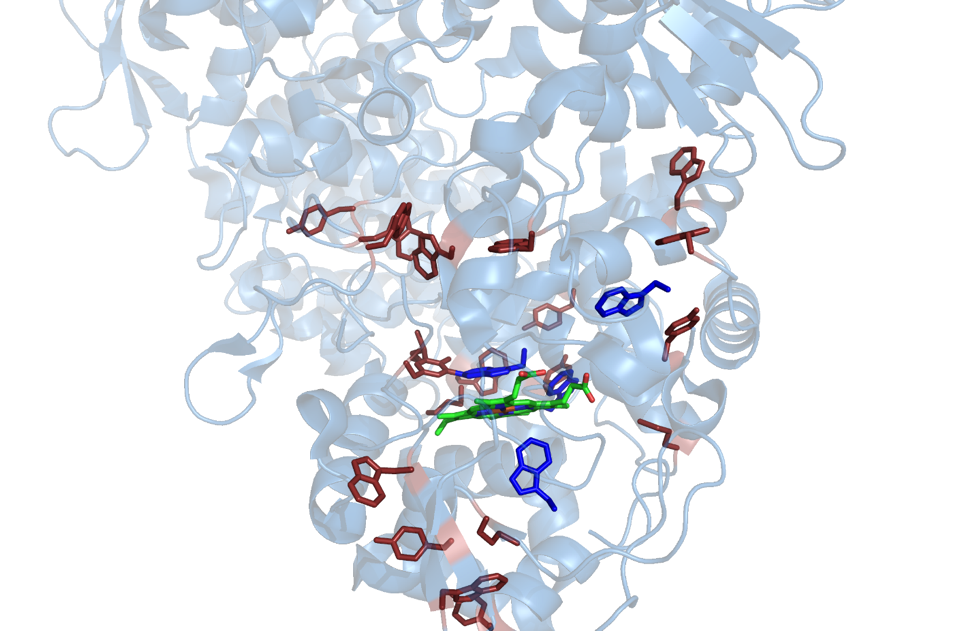
Figure 1. Possible hole-hopping routes from the KatG active site. Blue amino acids indicate possible starting points for hole-hopping routes, while red amino acids indicate possible continuations of each route.
Last Modified: