By: Natasha Narayanan, Bradley L. Merner
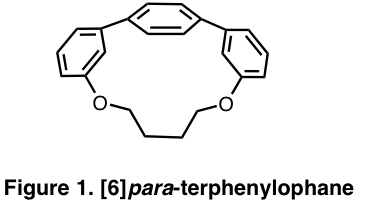
[n]para-terphenylophanes are compounds that contain three benzene rings linked at the para, or most remote, positions (Figure 1). The two outer (terminal) benzene rings are connected by a “tether” consisting of a bridge of atoms, where [n] refers to the number of atoms in the tether. Due to this tether connecting the two terminal benzene rings, the central benzene ring of the para-terphenyl unit is distorted out of planarity. Decreasing the number of atoms in the bridge shortens the tether, which increases the distortion of the central benzene ring and the strain energy of the molecule. Our group has developed a short, efficient synthesis of a series of para-terphenylophanes with varying tether lengths. We have recently turned our efforts towards the regioselective functionalization of these compounds for two reasons. First, in order to use these para-terphenylophanes as templates in the bottom-up synthesis of carbon nanotubes, functional group handles must be installed on the benzene rings to perform reactions necessary for elongation in the vertical direction. Secondly, a bent phenol moiety is present in the structure of a natural product known as haouamine A, which was isolated from a tunicate off the coast of Spain in 2003 and has shown promising anticancer activity. We hope to apply the synthetic methodology developed by our group for making bent functionalized arenes to the total synthesis of haouamine A, and in particular to the construction of the macrocyclic core containing the nonplanar phenol.
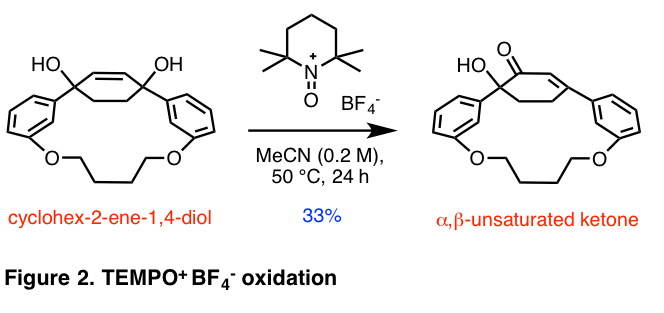
Beginning with a cyclohex-2-ene-1,4-diol, which serves as a bent precursor to the aromatized system in our synthesis of para-terphenylophanes, we envisioned that we could obtain the α,β-unsaturated ketone from the oxidation of the cyclohex-2-ene-1,4-diol in a [3.3] sigmatropic rearrangement. The dehydration of this α,β-unsaturated ketone would yield the nonplanar phenol. Various oxidation procedures reported in the literature to achieve tertiary allylic alcohol oxidation in a [3.3] sigmatropic rearrangement were tested, including PCC oxidation, IBX oxidation, and DMP oxidation, with no success. The desired transformation was achieved using a literature procedure that employs a TEMPO tetrafluoroborate salt (Figure 2).1 Attempts to aromatize the compound by eliminating a molecule of water have so far been unsuccessful. Future work will involve improving the yield of the TEMPO oxidation reaction, finding reaction conditions to aromatize the central benzene ring in order to obtain the nonplanar phenol, and investigating other synthetic routes to bent functionalized aromatic rings.
Recently, some exciting advancements have been made regarding the regioselective functionalization of the two terminal benzene rings of [n]para-terphenylophanes. We predicted that the two tertiary alcohols present in the cyclohex-2-ene-1,4-diol could be used as directing groups to direct the C-H functionalization of the terminal benzene rings. Based on a literature procedure, a tertiary alcohol-directed C-H functionalization reaction was used to successfully install a phenyl ring on one of the terminal benzene rings of a para-terphenylophane (Figure 3).2 This regioselective C-H functionalization chemistry can be used as a way to extend para-terphenylophanes into templates of sidewalls of carbon nanotubes. Future work will involve continued efforts to regioselectively functionalize both the central and terminal benzene rings of para-terphenylophanes, and use these installed functional groups as handles with which to extend the curved aromatic system present in para-terphenylophanes.

References
- Shibuya, M.; Tomizawa, M.; Iwabuchi, Y. Org. Chem. 2008, 73, 4750–4752.
- Terao, Y.; Wakui, H.; Nomoto, M.; Satoh, T.; Miura, M.; Nomura, M. Org. Chem. 2003, 68, 5236-5243.
Statement of Research Advisor
Natasha has been developing new synthetic strategies for oxidizing macrocyclic cyclohex-2-ene-1,4-diols into strained arene-bridged phenol units. These methods, if successful, will be of great importance tin developing a novel class of anticancer compounds, and functionalized benzeoind macrocycles that will be employed in the bottom-up chemical synthesis of carbon nanotubes and related substructures. Natasha has single-handedly completed the first step in this challenging process
—Bradley Merner, Chemistry
Last Modified: