By: Taylor Farmer, Peter Livant, Xiaoxun Li
A central challenge to synthetic organic chemists is forming a bond between two carbon atoms. The ability to form carbon-carbon bonds allows one to join together, with precise control, two smaller molecules to form a larger, more complex product. This is a core activity of the synthetic organic chemist.
In our laboratory, we needed compound 2 (Figure 1). Our original method of synthesizing 2 involved three separate reactions, starting with compound 1 (i.e., path a). Our group discovered that 1 could be converted to 2 in one step rather than three by treatment with an excess of sodium hydride (path b). This new reaction is termed a “cascade” because sequential reactions (here three) occur in one reaction vessel.
The cascade reaction forms a carbon-carbon double bond, highlighted in green in Figure 1. Reactions that form a carbon-carbon double bond by joining together previously separate molecules (rather than converting a single bond to a double bond within a molecule) are a somewhat rare type of reaction and quite important in organic chemistry. Examples of this type of reaction are the olefin-forming metathesis reaction, the Wittig reaction, and the McMurry reaction.
Due to the importance of forming a carbon-carbon double bond during our reaction, we strove to understand it in more detail. One means of testing how a new reaction functions is by testing its scope, i.e. the range of materials that can take part in the reaction successfully. One learns what structural changes help or hinder the reaction. At this point only compound 1 had been used in the cascade reaction. We chose three “bromo-nitro” compounds structurally similar to compound 1, viz. 4, 5, and 6 (Figure 1). Like compound 1, all have a bromo (Br) and a nitro (NO2) group attached to a single carbon in the molecule. After using each of these as the starting material for our cascade reaction, the product yields were found to range from 16-47%. These yields are comparable to those obtained with 1, namely 55% yield.
To make a bromo-nitro compound, a bromo-nitroso compound must be made first. An example is compound 3 (Figure 1) in which a nitroso group (NO) replaces a nitro group (NO2). Due to the similarity in structure, we proposed that these could be used as cascade starting materials as well. While results with bromo-nitroso compounds are preliminary, we have at least one case in which the carbon-carbon double bond formed. This means that we discovered a new reaction, which will be studied further.
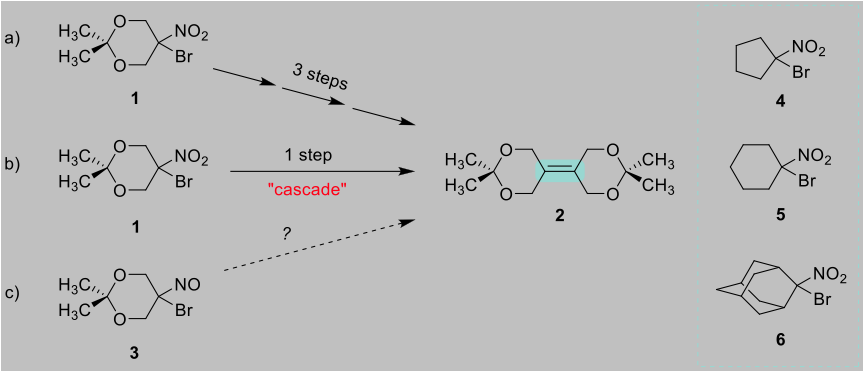
Figure 1. The cascade reaction and bromo-nitro compounds.
Statement of Research Advisor
Taylor has provided much needed empirical confirmation that the cascade reaction has broader applicability than the single example that existed before she began. Much of her effort was spent finding and perfecting a general method for synthesis of bromo-nitro compounds. That work led to the idea of trying bromo-nitroso compounds in the cascade reaction, an idea that has yielded positive preliminary results. – Peter Livant, Chemistry & Biochemistry
Last Modified: