By: Fraser Bronston, Christian R. Goldsmith
Catalysts that allow common synthetic reactions to take place in water instead of the usual organic solvents have long been sought after because of their potential to offset both the cost and the environmental impact of research and industry. The majority of catalysts used in modern synthetic chemistry fall victim to unfavorable physical and chemical interactions when exposed to water, which render them ineffective. Furthermore, the few compounds that are capable of catalyzing useful reactions in water often rely on chemical species whose actively catalytic forms are transient and poorly characterized, are inactivated by the presence of certain common functional groups, or show poor selectivity for their target reaction.
Our recent work has shown that two gallium(III)-based complexes, [Ga(phen)2Cl2]Cl (A) and [Ga(bispicen)Cl2]Cl (B), are capable of catalyzing the epoxidation of alkenes by peracetic acid in both water and acetonitrile, showing exceptional selectivity for the epoxide in both environments with no observed side products. Further investigation of aqueous activity in buffered solutions showed that both catalysts are equally effective under highly acidic and basic conditions, but nearly completely inactive in the near-neutral pH range. Functional group tolerance experiments conducted in acetonitrile suggest that alcohols, ketones, and organochlorides are not affected by the presence of the catalysts, but that amines and aldehydes might participate in unwanted side reactions.
Tuning reaction conditions in order to maximize product formation as simply as possible is a key aspect of catalysis research. As such, possible future directions for this research include searching for alternate terminal oxidants that allow the chemistry to be performed at neutral pH and a more thorough exploration of these catalysts’ interactions with aldehydes and amines.
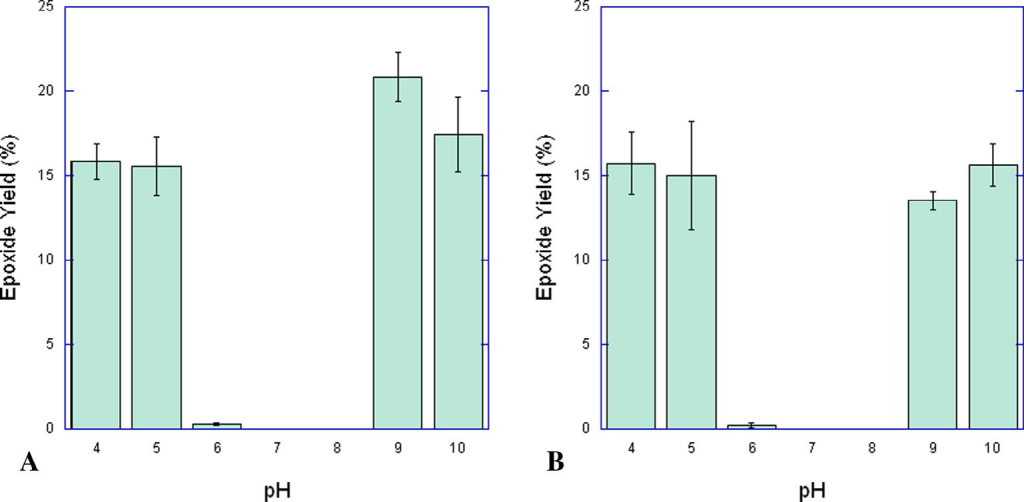
Figure 1. Yields of cyclohexene oxide generated in aqueous solutions at various constant pH values (A) Reaction conditions: [Ga(phen)2Cl2]Cl = 0.75 mM, [alkene] = 75.0 mM, [peracetic acid] = 151 mM. (B) [Ga(bispicen)Cl2]Cl = 0.85 mM, [alkene] = 85 mM, [peracetic acid] = 169 mM. Both series of experiments were performed under air at 25 °C.
Statement of Research Advisor
Fraser has contributed to the discovery of small molecule catalysts for hydrocarbon oxidation and the development of redox-responsive contrast agents for magnetic resonance imaging. He performed all of the catalytic reactions and data analysis and synthesized these two compounds when necessary.
—Christian R. Goldsmith, Chemistry and Biochemistry
Last Modified: